Trending...
- Crowdfunding Campaign Tips Off for 'NAWFSIDE' Short Film Highlighting Pressure in Youth Sports
- 'Wild Hermit Wellness' Has Achieved Bestseller Status in Just 2 Months Since Launch Of Organic Skincare Line
- Words of Veterans & Veterans Growing America Collaboration
FAYETTEVILLE, Ark. & AUCKLAND, New Zealand - PrAtlas -- Lineus Medical is pleased to announce a new distribution agreement with Venture Medical, a 100% New Zealand–owned medical supplier based in Auckland. Venture Medical specializes in vascular access and wound care and will now add Lineus Medical's SafeBreak® Vascular, the only breakaway device for IV lines clinically proven to reduce multiple IV complications, to its portfolio. This partnership expands SafeBreak's availability to hospitals and healthcare providers throughout New Zealand.
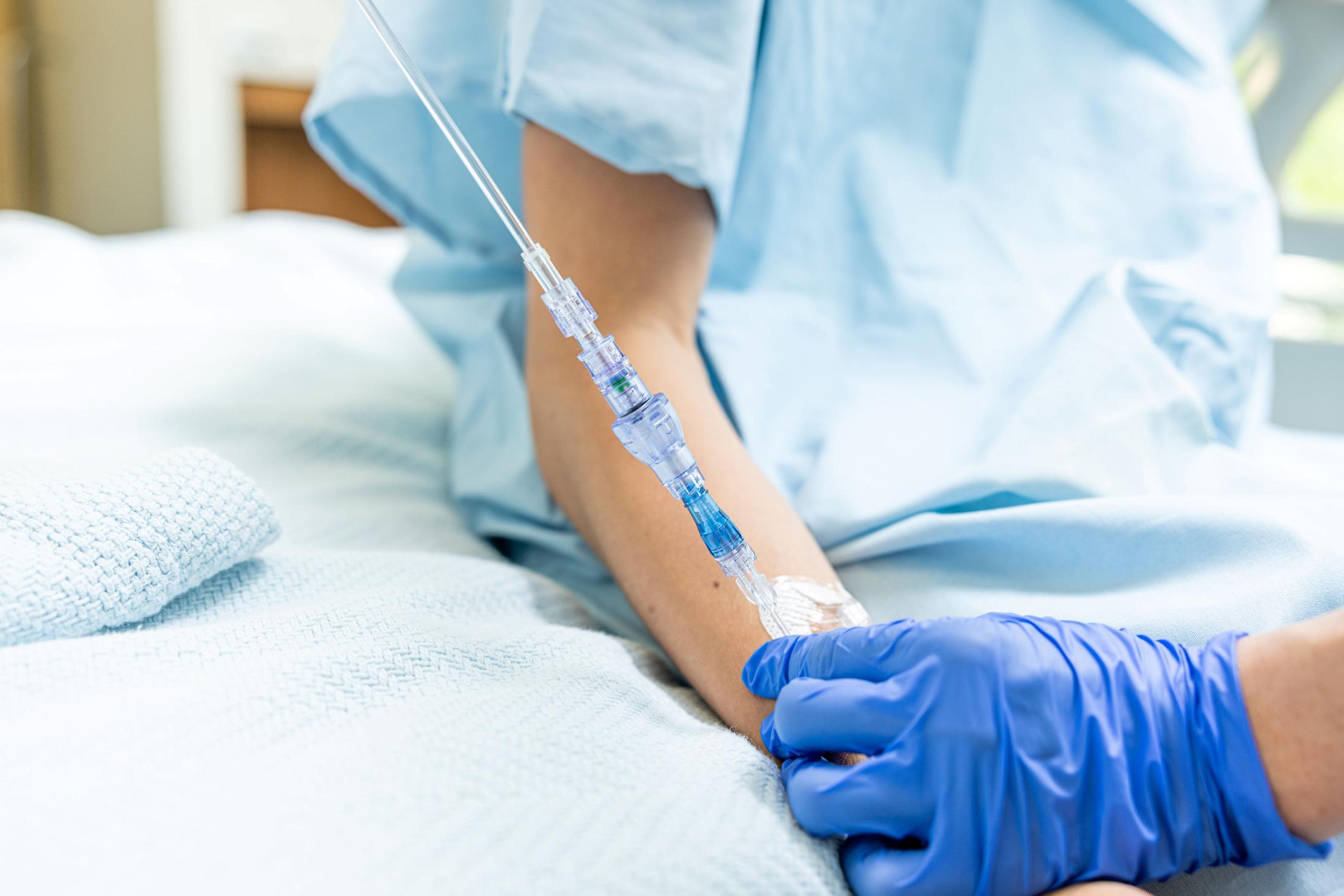
SafeBreak Vascular is the only breakaway device for IV lines clinically proven to reduce multiple IV complications.1 When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. The separated device also causes the IV pump to alarm, notifying the nursing staff to check on the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patient's infusion is restarted. It's a win-win-win. Patients avoid additional needlesticks, nurses save time, and hospitals save money1. SafeBreak is cleared for all vascular lines down to two weeks of age both in the United Sates and Canada.
More on PrAtlas
Larry Hayes, Lineus Medical's Chief Commercial Officer, stated, "This agreement marks a significant milestone in Lineus Medical's international growth. By partnering with Venture Medical, we are expanding SafeBreak Vascular into a new market and building momentum for broader global adoption. Venture Medical's local expertise and strong customer relationships make them the right partner to drive commercial success in New Zealand."
"Signing this agreement with Venture Medical is an important step in bringing SafeBreak Vascular to in the people of New Zealand.", Vance Clement CEO of Lineus Medical stated. "Our mission is to reduce the "pains" associated with medical lines and make SafeBreak the standard of care globally."
ABOUT LINEUS MEDICAL
Lineus Medical is the developer of a break-away technology that is proven to reduce IV restarts and IV complications1. Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found on the company website: www.lineusmed.com or you can follow the company on Facebook or LinkedIn.
More on PrAtlas
References
1. Data on file
SafeBreak Vascular is the only breakaway device for IV lines clinically proven to reduce multiple IV complications.1 When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. The separated device also causes the IV pump to alarm, notifying the nursing staff to check on the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patient's infusion is restarted. It's a win-win-win. Patients avoid additional needlesticks, nurses save time, and hospitals save money1. SafeBreak is cleared for all vascular lines down to two weeks of age both in the United Sates and Canada.
More on PrAtlas
- Pastor Darrell Armstrong Suspends Gubernatorial Campaign And Endorses Mikie Sherrill
- Dr. Johnny Shanks Attends Full Arch Growth Conference 2025
- Offline Asset Protection: NJTRX Implements 98 Percent Cold Storage as Industry Faces 2 Billion USD Losses
- Thousands of Smiles, Millions of Logo Views: RoarFun Brings Emotions Into Premium Retail Spaces with Formula Simulator for Immersive Brand Activation
- Qvarz LLC Expands Global Reach with High-Precision Quartz Cuvettes and Optical Components
Larry Hayes, Lineus Medical's Chief Commercial Officer, stated, "This agreement marks a significant milestone in Lineus Medical's international growth. By partnering with Venture Medical, we are expanding SafeBreak Vascular into a new market and building momentum for broader global adoption. Venture Medical's local expertise and strong customer relationships make them the right partner to drive commercial success in New Zealand."
"Signing this agreement with Venture Medical is an important step in bringing SafeBreak Vascular to in the people of New Zealand.", Vance Clement CEO of Lineus Medical stated. "Our mission is to reduce the "pains" associated with medical lines and make SafeBreak the standard of care globally."
ABOUT LINEUS MEDICAL
Lineus Medical is the developer of a break-away technology that is proven to reduce IV restarts and IV complications1. Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found on the company website: www.lineusmed.com or you can follow the company on Facebook or LinkedIn.
More on PrAtlas
- $300 Million Web3 Initiative and ZIGChain Partnership Power $20 Target in Noble Capital Markets Report for SEGG Media (N A S D A Q: SEGG)
- Assent Recognizes Manufacturers for Leading Supply Chain Sustainability Programs
- Arc Longevity Sells Out Debut Women's Creatine Gummy
- New Research Reveals Mild Cold—Not Extreme Cold—Delivers Real Health Benefits of Cold Therapy
- Phinge, Home of Netverse, Through its Extensive Software & Hardware Patent Portfolio, Shows Founder & CEO Robert DeMaio's Vision & Innovation
References
1. Data on file
Source: LineusMedical
0 Comments
Latest on PrAtlas
- He Started a New Career at 77; Maybe Not His Last
- "The Art of Philanthropy" — A Year-Long Campaign Supporting the USO and Military Veterans
- TRUE Palliative Care Launches as California Strengthens Commitment to Compassionate Care Under SB 403
- Mysterious Interstellar Object 3I/ATLAS Appears to Pause Near Mars, Exhibiting Periodic Light Pulses
- $73.6 Million in Order Backlog Poised for Explosive Growth in 2026; Streamlined Share Structure: Cycurion, Inc. (N A S D A Q: CYCU) $CYCU
- Osric Langevin Unveils "Quantitative Trend" Framework for Multi-Asset Analysis in Q4 2025
- Experience Days Named Among the UK's Top Christmas Gifts
- New Free Educational Bingo Cards Make Learning English Fun for First Graders
- Wzzph Provides Stablecoin Trading Solutions for Latin American Traders Amid Digital Currency Policy Adjustments
- NaturismRE Calls for Recognition of AI as Sentient Kin in Global Bill of Rights
- PDS Plumbing & Air Honors Veterans with "Free Tune-Up & A Turkey" Giveaway
- AgeImmune Announces the Launch of ImmuneG.I. — A Doctor-Formulated Herbal Supplement Supporting Gut and Digestive Wellness
- Precision Adult Care Unveils Essential Guidelines for Choosing a Senior Home Care Company
- Postmortem Pathology Delivers Expert Private Autopsy Services with Compassion and Precision
- Colorado Families Turn to Private Autopsies for Peace of Mind
- $5.4 Million Growth Acceleration, Fleet Expansion and $1.485 Million Strategic Financing: Multi Ways Holdings (N Y S E: MWG) $MWG
- Delta Capital Group Expands Business Funding Terms Up to 24 Months
- Hip-HopVibe.com Launches HHV Media Network in Partnership with The Publisher Desk
- CCHR: Misinformation Clouds Debate on Psychiatric Drug Toxicology Transparency
- Hilton Head Realtor becomes Certified Senior Professional