Trending...
- myLAB Box Expands, Becoming the First and Only At-Home Testing Company to Serve the Entire Family—Human and Furry—with New Pet Intolerance Test
- Tiger-Rock Martial Arts Appoints Jami Bond as Vice President of Growth
- Anderson Periodontal Wellness Attends 5th Joint Congress for Ceramic Implantology
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Refiles Abbreviated New Drug Application; $40 Price Target in New H. C. Wainright Research Report
MIAMI - PrAtlas -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression with Suicidality; Assuming Coverage with Buy and $40 Price Target.
Re-Filing of Abbreviated New Drug Application (ANDA) for KETAFREE™, Preservative-Free IV Ketamine.
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine.
Current Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
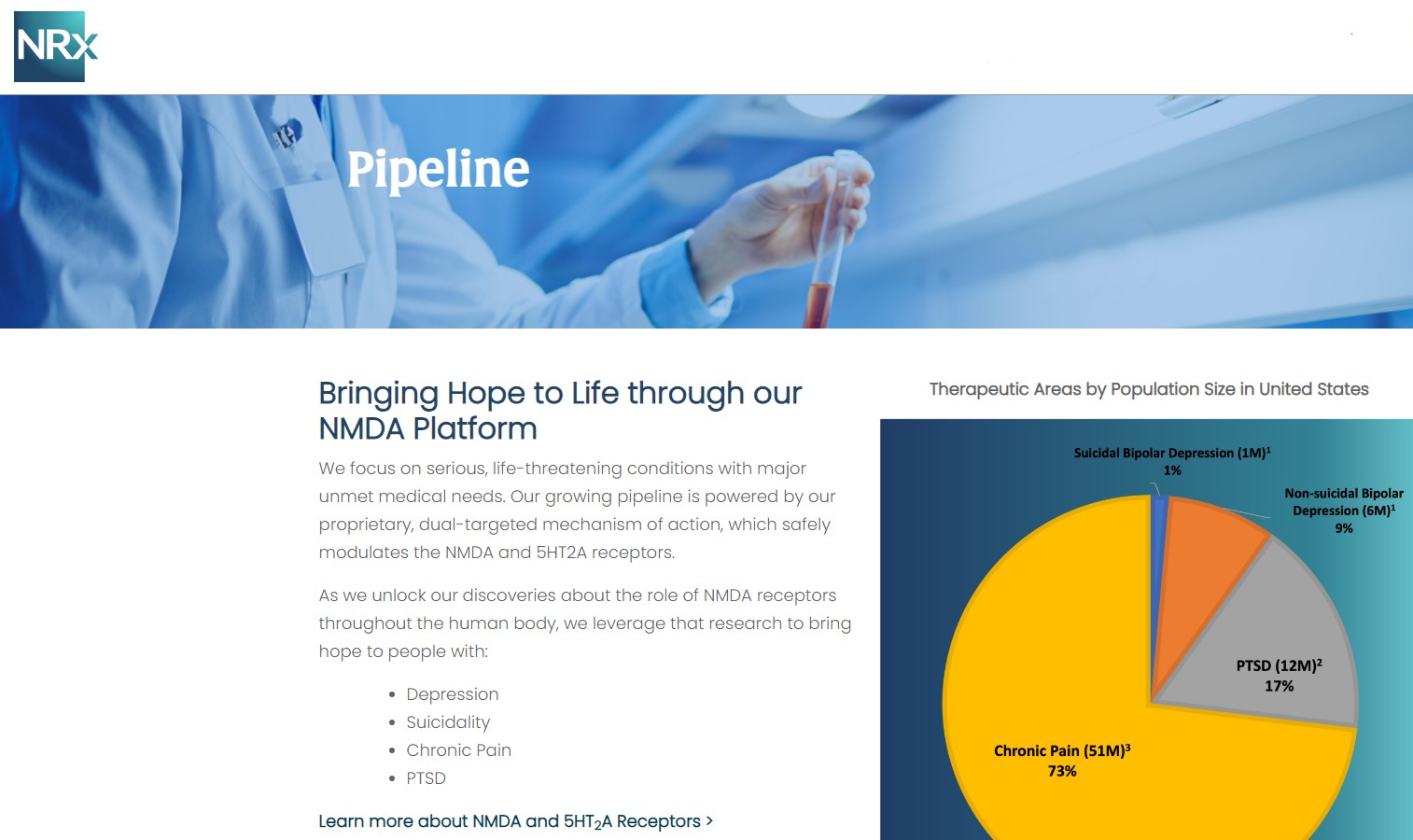
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
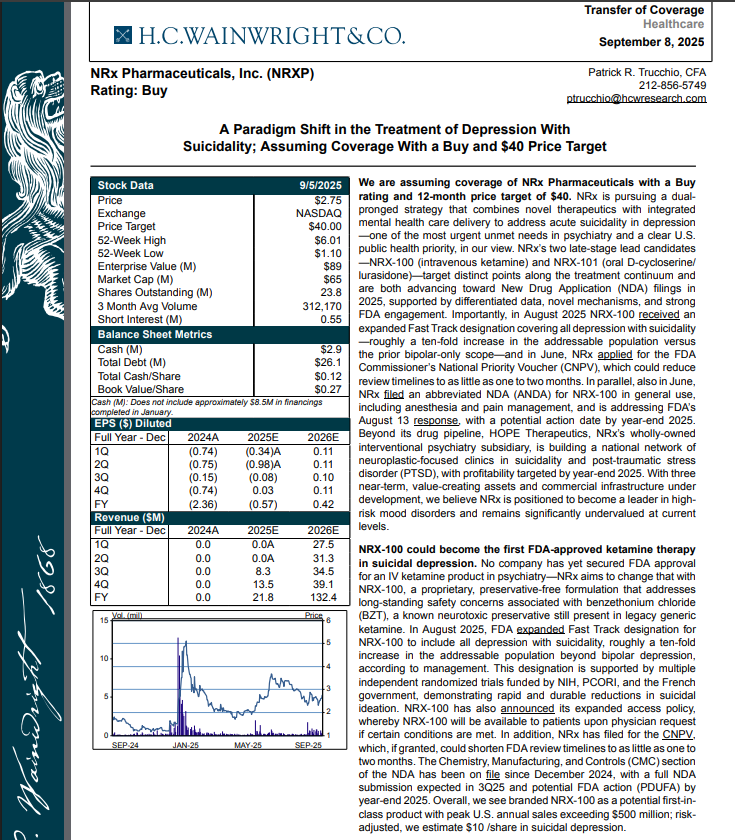
H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
More on PrAtlas
NRXP Re-Files Abbreviated New Drug Application (ANDA) for KETAFREE™, Preservative-Free IV Ketamine
On September 29th NRXP announced the re-filing of its Abbreviated New Drug Application (ANDA) to the U.S. Food and Drug Administration (FDA) for KETAFREE™, its preservative-free IV ketamine formulation, for use in all existing approved indications. The filing follows FDA grant of approval of its Suitability Petition for the NRXP proposed strength of preservative-free ketamine.
The current annual ketamine market is estimated at $750 million, with global demand for ketamine projected to grow to $3.35 billion by 2034. This does not include the widespread use of compounded ketamine by clinics unable to obtain manufactured drug. NRx aims to capture a significant share of the current market. According to a 2021 survey, an estimated 5.1 million Americans had received ketamine for medical uses in their lifetime3, a number that continues to grow with increased clinical focus on this important medication. Ketamine currently faces a severe drug shortage according to the American Society of Hospital Pharmacists. Accordingly, NRXP is seeking priority review from FDA.
NRXP previously filed a citizen's petition with the FDA to remove benzethonium chloride (BZT), a known neurotoxic and cytotoxic substance, from presentations of ketamine intended for intravenous use. The FDA has previously disallowed the use of BZT in hand cleansers and topical antiseptics. A related preservative, benzalkonium chloride has demonstrated corneal and conjunctival toxicity in artificial tears and glaucoma medications, leading to use of preservative-free alternatives. NRXP has filed expert testimony from accredited toxicologists regarding the toxicity of BZT, which is not generally recognized as safe (GRAS) by the FDA. Removal of potentially harmful preservatives from foods is a stated priority in the MAHA report and HHS leadership has additionally targeted preservatives in vaccines. BZT was originally added to ketamine when it was first formulated in the 1970s to maintain stability and sterility using the container closure systems then available. NRx has demonstrated long term stability and sterility with a patented preservative-free formulation using modern manufacturing methods.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
More on PrAtlas
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Second Quarter 2025 Corporate Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents.
The latest NRXP key developments included the following points:
NRXP Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression with Suicidality; Assuming Coverage with Buy and $40 Price Target.
Re-Filing of Abbreviated New Drug Application (ANDA) for KETAFREE™, Preservative-Free IV Ketamine.
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine.
Current Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
More on PrAtlas
- Crunchbase Ranks Phinge Founder & CEO Robert DeMaio #1 Globally. Meet him in Las Vegas-Week of CES to Learn About Netverse, Patented App-less Platform
- IODefi Introduces New Web3 Infrastructure Framework as XRP Ledger Development Gains Global Attention
- Terizza Forms Strategic Collaboration with UC San Diego to Pioneer Next-Generation Distributed AI Infrastructure
- EnergyStrat Launches Global LNG Risk Outlook 2025–2030
- Strong Revenue Gains, Accelerating Growth, Strategic Hospital Expansion & Uplisting Advancements: Cardiff Lexington Corporation (Stock Symbol: CDIX)
NRXP Re-Files Abbreviated New Drug Application (ANDA) for KETAFREE™, Preservative-Free IV Ketamine
On September 29th NRXP announced the re-filing of its Abbreviated New Drug Application (ANDA) to the U.S. Food and Drug Administration (FDA) for KETAFREE™, its preservative-free IV ketamine formulation, for use in all existing approved indications. The filing follows FDA grant of approval of its Suitability Petition for the NRXP proposed strength of preservative-free ketamine.
The current annual ketamine market is estimated at $750 million, with global demand for ketamine projected to grow to $3.35 billion by 2034. This does not include the widespread use of compounded ketamine by clinics unable to obtain manufactured drug. NRx aims to capture a significant share of the current market. According to a 2021 survey, an estimated 5.1 million Americans had received ketamine for medical uses in their lifetime3, a number that continues to grow with increased clinical focus on this important medication. Ketamine currently faces a severe drug shortage according to the American Society of Hospital Pharmacists. Accordingly, NRXP is seeking priority review from FDA.
NRXP previously filed a citizen's petition with the FDA to remove benzethonium chloride (BZT), a known neurotoxic and cytotoxic substance, from presentations of ketamine intended for intravenous use. The FDA has previously disallowed the use of BZT in hand cleansers and topical antiseptics. A related preservative, benzalkonium chloride has demonstrated corneal and conjunctival toxicity in artificial tears and glaucoma medications, leading to use of preservative-free alternatives. NRXP has filed expert testimony from accredited toxicologists regarding the toxicity of BZT, which is not generally recognized as safe (GRAS) by the FDA. Removal of potentially harmful preservatives from foods is a stated priority in the MAHA report and HHS leadership has additionally targeted preservatives in vaccines. BZT was originally added to ketamine when it was first formulated in the 1970s to maintain stability and sterility using the container closure systems then available. NRx has demonstrated long term stability and sterility with a patented preservative-free formulation using modern manufacturing methods.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
More on PrAtlas
- Holiday Decorations Most Likely to Cause Injuries
- UK Financial Ltd Confirms Official Corporate Structure of the Maya Preferred Project and Its Dual-Class Token System
- CCHR Florida Joins Global Call to Ban Electroshock Treatment, Citing New Evidence of Widespread Patient Harm
- BoxingRx Announces Full Gym Renovation Ahead of New Ownership's One-Year Anniversary
- UK Financial Ltd Announces It's Official Corporate Headquarters In The United Kingdom
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Second Quarter 2025 Corporate Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents.
The latest NRXP key developments included the following points:
NRXP Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
Filed Under: Health, Banking, Biotech, Stocks, Financial, Finance, Medical, Marketing, Media, Healthcare, Stock Market, Nasdaq
0 Comments
Latest on PrAtlas
- Coalition and CCHR Call on FDA to Review Electroshock Device and Consider a Ban
- Spark Announces 2025 Design Award Winners
- NEW Luxury Single-Family Homes Coming Soon to Manalapan - Pre-Qualify Today for Priority Appointments
- Dominic Pace Returns to the NCIS Franchise With Guest Role on NCIS: Origins
- Anderson Periodontal Wellness Attends 5th Joint Congress for Ceramic Implantology
- UK Financial Ltd Completes Full Ecosystem Conversion With Three New ERC-3643 SEC-Ready Tokens As MCAT Deadline Closes Tonight
- AI Real Estate Company Quietly Building a National Powerhouse: reAlpha Tech Corp. (N A S D A Q: AIRE)
- Inkdnylon Expands National Uniform Embroidery Services
- Appliance EMT Expands Appliance Repair Services to Portland, OR and Vancouver, WA
- Next Week: The World's Best Young Pianists Arrive in Music City for the 2025 Nashville International Chopin Piano Competition
- Revenue Optics Builds Out Its Dedicated Sales Recruiting Firm with Strategic Addition of Christine Schafer
- Hydrofast Elevates the Holiday Season: The C100 Countertop RO System Merges Smart Tech with Wellness for the Perfect Christmas Gift
- Melospeech Inc. Accepts Nomination for HealthTech Startup of the Year
- Flower City Tattoo Convention Draws Record Attendance in Rochester, NY
- KIKO NATION TOKEN (Official Release)
- Verb™ Presents Features Vanguard Personalized Indexing: Utilizing Advanced Tax-Loss Harvesting Technology
- UK Financial Ltd Announces A Special Board Meeting Today At 4PM: Orders MCAT Lock on CATEX, Adopts ERC-3643 Standard, & Cancels $0.20 MCOIN for $1
- 6 Holiday Looks That Scream "Old Money" But Cost Less Than Your Christmas Tree
- From Cheer to Courtroom: The Hidden Legal Risks in Your Holiday Eggnog
- Controversial Vegan Turns Rapper Launches First Song, "Psychopathic Tendencies."