Trending...
- Georgia's Lanier Islands Resort Tees Up for a New Era of Golf in Spring 2026
- InspireTech Global and SKADI Cyber Defense Announce Strategic Partnership to Deliver Autonomous Cybersecurity to Canadian Education and Public Sector
- Crossroads4Hope Kicks Off Its 25th Year of Caring with the Launch of Free Breast and Colorectal Cancer Resources for Patients and Families Nationwide
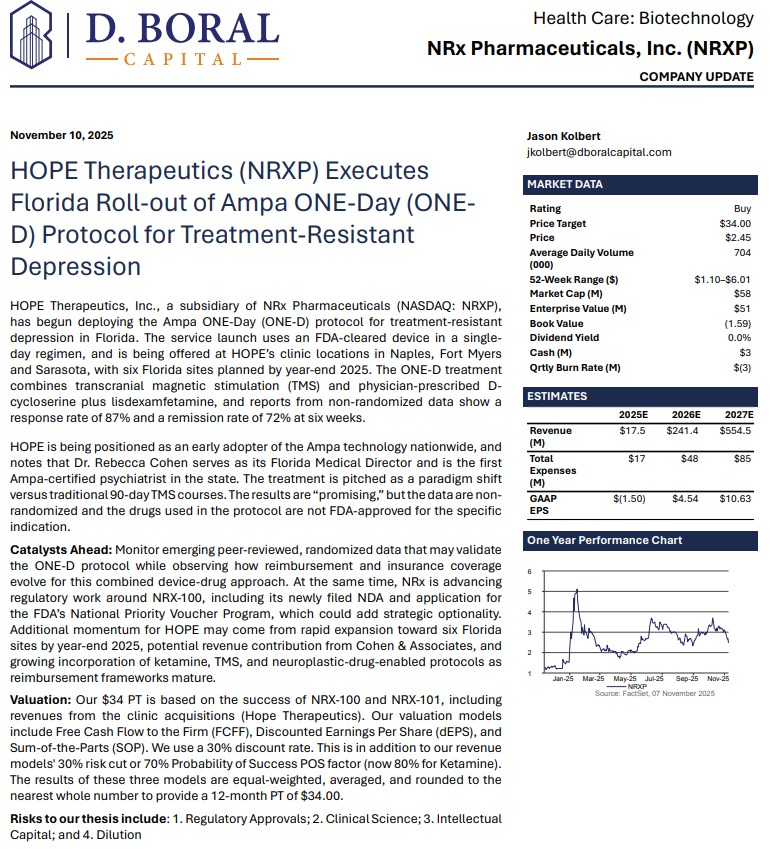
H.C. Wainwright Initiates Coverage with $34 Price Target, Citing Paradigm Shift in Depression Treatment. NRx Pharmaceuticals (N A S D A Q: NRXP) $NRXP
MIAMI - PrAtlas -- NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP), a clinical-stage biopharmaceutical company pioneering NMDA-based therapeutics for central nervous system disorders, is emerging as a potential leader in the next generation of depression and suicidality treatments. With its innovative ONE-D (One Day Depression) protocol now launched in Florida and a $40 price target set by H.C. Wainwright, NRXP appears positioned for transformational growth in a rapidly expanding $3.35 billion global market.
A Major Step Forward: ONE-D Depression Treatment Launches in Florida
NRx Pharmaceuticals has initiated the first-in-Florida rollout of the ONE-D (One Day) Depression Treatment, in partnership with Ampa Health. The innovative protocol has shown breakthrough potential in achieving remission from treatment-resistant depression within a single day—an unprecedented leap forward for millions suffering from chronic depression.
Unlike traditional transcranial magnetic stimulation (TMS) therapies requiring up to 90 sessions over three months, the ONE-D approach combines a single-day TMS treatment with physician-prescribed compounds, including D-cycloserine, an active component of NRx's investigational NRX-101, and lisdexamfetamine. Early clinical data show an 87% response rate and 72% remission rate in nonrandomized studies.
The Ampa device is now available across NRXP HOPE Clinics in Sarasota, Naples, and Fort Myers, Florida—with six total locations expected by the end of 2025. This initiative marks one of the first national deployments of the Ampa ONE-D protocol.
More on PrAtlas
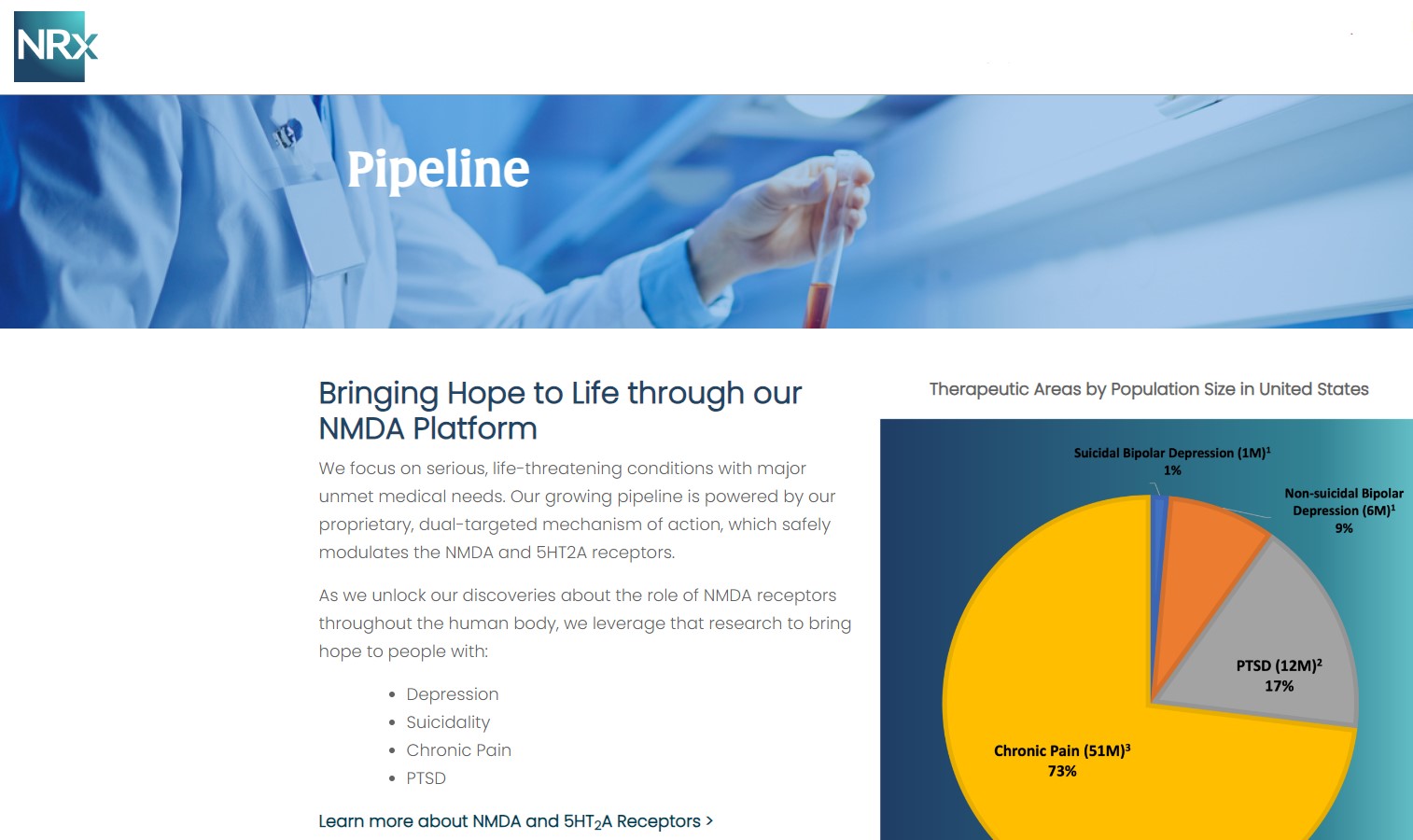
Breakthrough Therapeutic Portfolio: NRX-101 and KETAFREE™
At the center of NRXP's drug pipeline is NRX-101, an FDA-designated Breakthrough Therapy targeting suicidal, treatment-resistant bipolar depression and chronic pain. The drug represents a potentially lifesaving option for the more than 13 million Americans who seriously consider suicide each year, according to the CDC.
NRXP is also advancing KETAFREE™, a preservative-free IV ketamine formulation, through a refiled Abbreviated New Drug Application (ANDA) with the FDA. This comes as the ketamine market—currently valued at $750 million—is projected to reach $3.35 billion globally by 2034.
Importantly, the FDA has granted approval of NRXP's Suitability Petition for its proposed strength of preservative-free ketamine, clearing the path for potential market entry amid ongoing national shortages of sterile ketamine formulations.
Strategic Expansion Through Dura Medical Acquisition
NRXP recently completed its acquisition of Dura Medical, a revenue-generating and EBITDA-positive network of precision psychiatry clinics along Florida's West Coast. The addition of Dura, alongside pending acquisitions such as Neurospa TMS and Cohen & Associates, positions NRXP to deliver an integrated continuum of mental health care spanning TMS, ketamine therapy, and advanced pharmacologic treatments for PTSD, depression, and chronic pain.
Robust Financial and Partnership Outlook
The company's growth strategy is supported by a $7.8 million debt financing deal with Universal Capital, LLC, aimed at expanding NRXP's HOPE Clinic network. In parallel, NRXP has accepted non-binding potential licensing terms for NRX-100, with the agreement representing over $300 million in milestone payments and tiered double-digit royalties—a major value inflection opportunity for investors.
More on PrAtlas
Moreover, NRXP's partnership with Alvogen Pharmaceuticals supports the ongoing development and commercial readiness of NRX-101, further validating the company's clinical and commercial trajectory.
Analyst Confidence: H.C. Wainwright Issues "Buy" Rating and $40 Price Target
A recent analyst report from H.C. Wainwright & Co. titled "A Paradigm Shift in the Treatment of Depression with Suicidality" initiated coverage on NRx Pharmaceuticals with a Buy rating and a $34 price target. The report underscores NRXP's leadership in transforming how depression and suicidality are treated, leveraging FDA Fast Track and Breakthrough Therapy designations to accelerate commercialization.
"We view NRXP's approach as a paradigm shift in the treatment of depression with suicidality," wrote H.C. Wainwright analysts. "With novel drug candidates, a rapidly expanding clinic network, and strategic licensing opportunities, NRXP is uniquely positioned to capture substantial market share."
About NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
NRx Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company developing breakthrough therapeutics based on its NMDA receptor modulation platform for the treatment of central nervous system disorders, including suicidal bipolar depression, chronic pain, and PTSD.
Through its partnerships, innovative clinical pipeline, and integrated treatment model, NRXP is setting new standards in precision psychiatry and interventional mental health care.
For More Information:
Company: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com
Website: www.nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
A Major Step Forward: ONE-D Depression Treatment Launches in Florida
NRx Pharmaceuticals has initiated the first-in-Florida rollout of the ONE-D (One Day) Depression Treatment, in partnership with Ampa Health. The innovative protocol has shown breakthrough potential in achieving remission from treatment-resistant depression within a single day—an unprecedented leap forward for millions suffering from chronic depression.
Unlike traditional transcranial magnetic stimulation (TMS) therapies requiring up to 90 sessions over three months, the ONE-D approach combines a single-day TMS treatment with physician-prescribed compounds, including D-cycloserine, an active component of NRx's investigational NRX-101, and lisdexamfetamine. Early clinical data show an 87% response rate and 72% remission rate in nonrandomized studies.
The Ampa device is now available across NRXP HOPE Clinics in Sarasota, Naples, and Fort Myers, Florida—with six total locations expected by the end of 2025. This initiative marks one of the first national deployments of the Ampa ONE-D protocol.
More on PrAtlas
- Norisia Launches AI Formulated Luxury Multivitamin to Transform Daily Wellness in the UK
- Jacob Emrani's Annual "Supper Bowl" Expected To Donate Thousands Of Meals
- NASA / Glenn Research Center Collaboration to Help Meet Rising Demand for Space Energy Beaming Tech / CIGS PV Modules from Ascent Solar: NAS DAQ: ASTI
- When Interpretation Becomes Conversation: Rethinking Engagement in the Museum Age
- Half of Finnish Online Gambling Expenditure Now Flows to Offshore Instant Casinos as License Applications Open March 1, 2026
Breakthrough Therapeutic Portfolio: NRX-101 and KETAFREE™
At the center of NRXP's drug pipeline is NRX-101, an FDA-designated Breakthrough Therapy targeting suicidal, treatment-resistant bipolar depression and chronic pain. The drug represents a potentially lifesaving option for the more than 13 million Americans who seriously consider suicide each year, according to the CDC.
NRXP is also advancing KETAFREE™, a preservative-free IV ketamine formulation, through a refiled Abbreviated New Drug Application (ANDA) with the FDA. This comes as the ketamine market—currently valued at $750 million—is projected to reach $3.35 billion globally by 2034.
Importantly, the FDA has granted approval of NRXP's Suitability Petition for its proposed strength of preservative-free ketamine, clearing the path for potential market entry amid ongoing national shortages of sterile ketamine formulations.
Strategic Expansion Through Dura Medical Acquisition
NRXP recently completed its acquisition of Dura Medical, a revenue-generating and EBITDA-positive network of precision psychiatry clinics along Florida's West Coast. The addition of Dura, alongside pending acquisitions such as Neurospa TMS and Cohen & Associates, positions NRXP to deliver an integrated continuum of mental health care spanning TMS, ketamine therapy, and advanced pharmacologic treatments for PTSD, depression, and chronic pain.
Robust Financial and Partnership Outlook
The company's growth strategy is supported by a $7.8 million debt financing deal with Universal Capital, LLC, aimed at expanding NRXP's HOPE Clinic network. In parallel, NRXP has accepted non-binding potential licensing terms for NRX-100, with the agreement representing over $300 million in milestone payments and tiered double-digit royalties—a major value inflection opportunity for investors.
More on PrAtlas
- RTC Communications Completes Next Level Connect Fiber Expansion Bringing Multi-Gig Broadband to West Boggs Community
- EPP Pricing Platform announces leadership transition to support long-term growth and continuity
- Stolen Hearts: Reclaiming Your Child From Parental Alienation (narcissistic abuse)
- Roshni Online Services Unveils Plans for Innovative Digital Consultation Platform
- Wall Street Is Missing This One: Cycurion (NAS DAQ: CYCU) Gets $7 Price Target While Trading at a Steep Discount
Moreover, NRXP's partnership with Alvogen Pharmaceuticals supports the ongoing development and commercial readiness of NRX-101, further validating the company's clinical and commercial trajectory.
Analyst Confidence: H.C. Wainwright Issues "Buy" Rating and $40 Price Target
A recent analyst report from H.C. Wainwright & Co. titled "A Paradigm Shift in the Treatment of Depression with Suicidality" initiated coverage on NRx Pharmaceuticals with a Buy rating and a $34 price target. The report underscores NRXP's leadership in transforming how depression and suicidality are treated, leveraging FDA Fast Track and Breakthrough Therapy designations to accelerate commercialization.
"We view NRXP's approach as a paradigm shift in the treatment of depression with suicidality," wrote H.C. Wainwright analysts. "With novel drug candidates, a rapidly expanding clinic network, and strategic licensing opportunities, NRXP is uniquely positioned to capture substantial market share."
About NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
NRx Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company developing breakthrough therapeutics based on its NMDA receptor modulation platform for the treatment of central nervous system disorders, including suicidal bipolar depression, chronic pain, and PTSD.
Through its partnerships, innovative clinical pipeline, and integrated treatment model, NRXP is setting new standards in precision psychiatry and interventional mental health care.
For More Information:
Company: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com
Website: www.nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
Filed Under: Health, Banking, Biotech, Stocks, Financial, Finance, Medical, Marketing, Media, Healthcare, Stock Market, Nasdaq
0 Comments
Latest on PrAtlas
- P‑Wave Classics Launches Definitive New Edition of Hannah Webster Foster's The Coquette
- Strategic Expansion with 3 New Alliances — Jefferson Beach Yacht Sales, CFR YS & flyExclusive Incentive Partnership: Off The Hook YS: (N Y S E: OTH)
- Super League (N A S D A Q: SLE) Advances AI-Driven Playable Media with AdArcade, Solsten, and Meta-Stadiums Partnerships, Plus Roblox Theatre Launch
- purelyIV Expands Concierge Wellness Platform with New IV Therapies, Memberships, and Digital Experience
- CCHR: Europe Rejects Forced Psychiatry—Landmark Vote Declares Coercive Practices Incompatible with Human Rights
- Crossroads4Hope Kicks Off Its 25th Year of Caring with the Launch of Free Breast and Colorectal Cancer Resources for Patients and Families Nationwide
- OpenSSL Corporation Advisory Committees' Elections 2026: Voting Now Open
- Good Vibes Club and Instant IP Forge Strategic Partnership to Secure IP Brand Value in a Booming Digital Economy
- Inkdnylon Simplifies Digitizing and Vector Art Nationwide With Clear Pricing and Guided File Support
- goldsilbermarkt.de Awarded "Business Champion" in Online Retail by DISQ
- InspireTech Global and SKADI Cyber Defense Announce Strategic Partnership to Deliver Autonomous Cybersecurity to Canadian Education and Public Sector
- Kaltra Expands Microchannel Innovation to Deliver Lower Refrigerant Charge
- Georgia's Lanier Islands Resort Tees Up for a New Era of Golf in Spring 2026
- Eagle Americas Expands Into the Western U.S. With High West Machine Tool
- Desert Mountain Club Earns Prestigious Blue Zones Approved™ Triple Designation, a New Standard for Well-Being in a Luxury Lifestyle Community
- Outsports announces record-breaking number of LGBTQ+ athletes at 2026 Milan Winter Olympics
- Sheffield Clinic Highlights Safe, Inclusive Laser Hair Removal While Improving Access
- Appliance EMT Partners with Kids Motel Ministry in Metro Atlanta
- CNCPW Divulga Dados de Liquidez do 1º Trimestre: Confirma 100% de Reservas e Atualiza Protocolos de "Saque CNCPW" Contra Fluxos Ilícitos
- Tech Workers Are Escaping "Forever Layoffs" By Becoming Their Own Boss