Trending...
- Fenix Consulting Group Expands Orange County Office to Meet Growing Client Demand
- Broadway Smile Boutique Unveils Modern Website for Enhanced Patient Experience
- CCHR: New Data Shows Millions of U.S. Children Caught in Escalating Psychiatric Polypharmacy
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Has $7.8 Million for Clinic Acquisitions and Purchase of Kadima Neuropsychiatry Institute as Treatment Model and Leading Investigative Site for Suicidal Depression / PTSD
MIAMI - PrAtlas -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need, Based on FDA's Assessment of Data Submitted.
13 Million Adults Seriously Consider Suicide Each Year, According to the CDC, 3.2 Million Make a Plan to Commit Suicide.
Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA).
Actions Taken to Request the Removal of Benzethonium Chloride from Ketamine Products in Favor of the Company's Safer and Superior Options.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
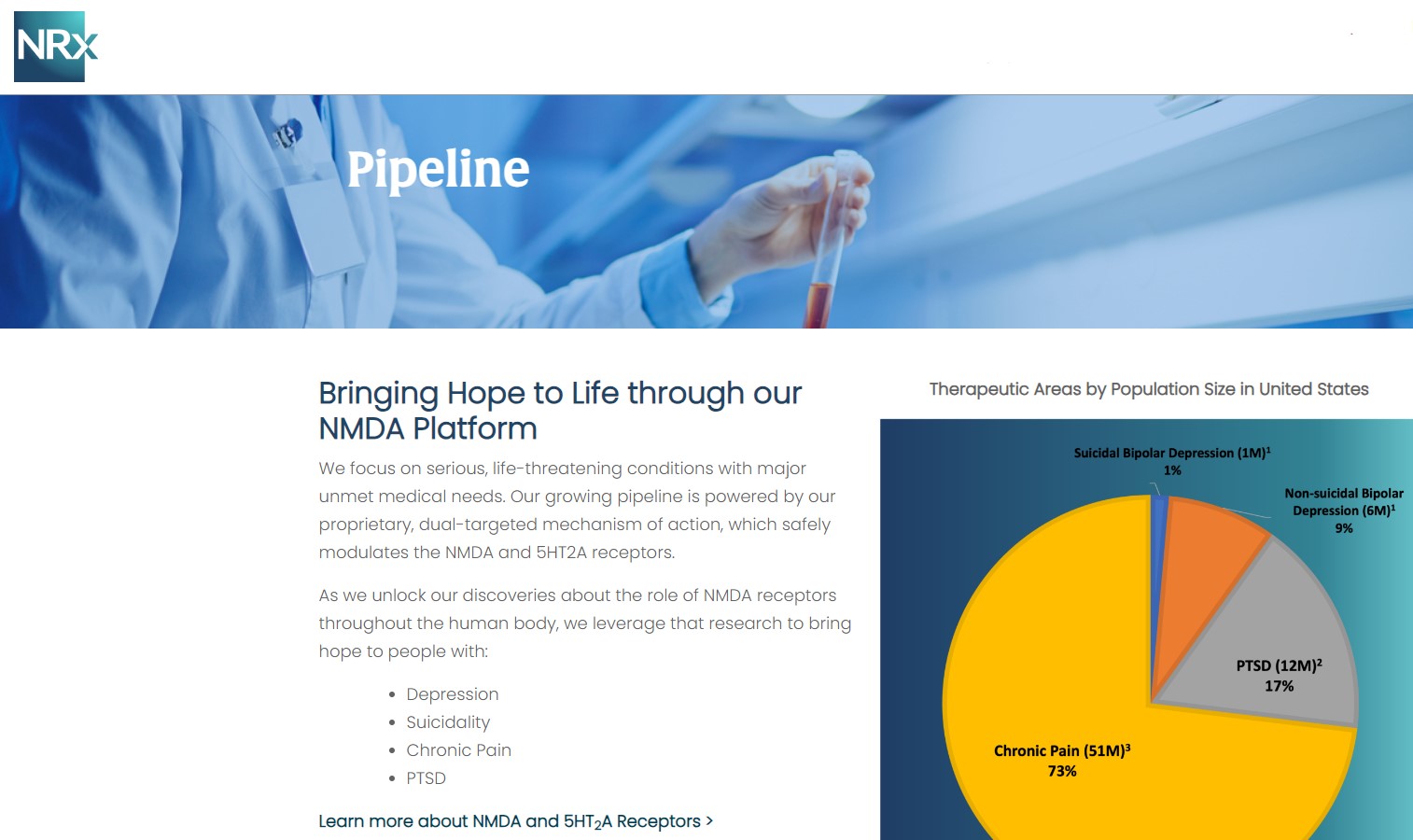
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
More on PrAtlas
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, including Bipolar Depression
On August 11th NRXP announced the US Food and Drug Administration (FDA) has granted Fast Track designation to its NRX-100 for the treatment of suicidal ideation in patients with depression, including bipolar depression. This designation for NRX-100 as a standalone drug is a 10-fold expansion of the addressable population for NRX-100, compared to the designation granted in 2017 for NRX-100 in combination with NRX-101 (DCS/lurasidone) for treatment of Suicidal Bipolar Depression.
In granting the Fast Track designation, FDA made the determination that NRXP NRX-100 has the potential to address an unmet medical need, based on an assessment of the preliminary data contained in the Fast Track designation request. This determination of unmet medical need aligns with the eligibility requirements for the Commissioner's National Priority Voucher Program and for the FDA's Accelerated Approval Program.
NRXP has applied for a CNPV, which has the potential to substantially shorten the review cycle for NRX-100. Several well-controlled trials submitted to FDA in support of Fast Track Designation demonstrated a clinically meaningful and statistically significant reduction of suicidal ideation.
Regarding Fast Track designation, FDA's website states: A drug that receives Fast Track designation is eligible for some or all of the following:
More frequent meetings with FDA to discuss the drug's development plan and ensure collection of appropriate data needed to support drug approval.
More frequent written communication from FDA about such things as the design of the proposed clinical trials and use of biomarkers
Eligibility for Accelerated Approval and Priority Review, if relevant criteria are met.
Rolling Review, which means that a drug company can submit completed sections of its Biologic License Application (BLA) or New Drug Application (NDA) for review by FDA, rather than waiting until every section of the NDA is completed before the entire application can be reviewed. BLA or NDA review usually does not begin until the drug company has submitted the entire application to the FDA.
NRXP NRX-100 is poised to address the >$3 billion Suicidal Depression market in the US.
Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA)
On August 8th NRXP announced it has received final clearance and approval from the Florida Agency for Health Care Administration (AHCA) to proceed closing of its Dura Medical LLC acquisition, in connection with its change of ownership applications, a key regulatory step for closing. Dura is revenue generating and EBITDA positive.
More on PrAtlas
Request to the Food and Drug Administration Seeking Removal of Benzethonium Chloride from Ketamine Products to Favor a Switch to Better Options
On August 4th NRXP announced its actions with the US Food and Drug Administration (FDA), seeking the removal of Benzethonium Chloride from ketamine sold in the United States. Benzethonium Chloride (BZT) is a preservative that is not Generally Recognized as Safe (GRAS) by the FDA for parenteral products and not Generally Recognized as Safe and Effective (GRASE) for topical products. The FDA no longer allows BZT to be used in hand cleansers and topical antiseptics and other products on the market today for safety concerns. The ketamine versions made by NRXP is BZT free and therefore presents a safer and superior option.
Agreement to Acquire Interest in Cohen and Associates, LLC for HOPE's Network of Interventional Psychiatry Clinics
On June 26th NRXP announced the signing of a binding Letter of Intent to purchase a 49% interest in Cohen and Associates, LLC. Cohen is expected to serve as a foundational clinic for NRXP in the Sarasota-Bradenton region of western Florida.
Filing of Commissioner's National Priority Voucher Application for Intravenous Ketamine (NRX-100)
On June 23rd NRXP announced filing for the newly-announced FDA Commissioner's National Priority Voucher program on behalf of NRX-100, its patent-pending, preservative-free formulation of ketamine for intravenous use.
Strategic Investor Relations Partnership with astr partners
On June 16th NRXP announced a strategic investor relations partnership with astr partners, a boutique investor relations and capital advisory firm focused on the life sciences sector.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need, Based on FDA's Assessment of Data Submitted.
13 Million Adults Seriously Consider Suicide Each Year, According to the CDC, 3.2 Million Make a Plan to Commit Suicide.
Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA).
Actions Taken to Request the Removal of Benzethonium Chloride from Ketamine Products in Favor of the Company's Safer and Superior Options.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
More on PrAtlas
- Inkdnylon Expands National Uniform Embroidery Services
- Appliance EMT Expands Appliance Repair Services to Portland, OR and Vancouver, WA
- Next Week: The World's Best Young Pianists Arrive in Music City for the 2025 Nashville International Chopin Piano Competition
- Revenue Optics Builds Out Its Dedicated Sales Recruiting Firm with Strategic Addition of Christine Schafer
- Hydrofast Elevates the Holiday Season: The C100 Countertop RO System Merges Smart Tech with Wellness for the Perfect Christmas Gift
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, including Bipolar Depression
On August 11th NRXP announced the US Food and Drug Administration (FDA) has granted Fast Track designation to its NRX-100 for the treatment of suicidal ideation in patients with depression, including bipolar depression. This designation for NRX-100 as a standalone drug is a 10-fold expansion of the addressable population for NRX-100, compared to the designation granted in 2017 for NRX-100 in combination with NRX-101 (DCS/lurasidone) for treatment of Suicidal Bipolar Depression.
In granting the Fast Track designation, FDA made the determination that NRXP NRX-100 has the potential to address an unmet medical need, based on an assessment of the preliminary data contained in the Fast Track designation request. This determination of unmet medical need aligns with the eligibility requirements for the Commissioner's National Priority Voucher Program and for the FDA's Accelerated Approval Program.
NRXP has applied for a CNPV, which has the potential to substantially shorten the review cycle for NRX-100. Several well-controlled trials submitted to FDA in support of Fast Track Designation demonstrated a clinically meaningful and statistically significant reduction of suicidal ideation.
Regarding Fast Track designation, FDA's website states: A drug that receives Fast Track designation is eligible for some or all of the following:
More frequent meetings with FDA to discuss the drug's development plan and ensure collection of appropriate data needed to support drug approval.
More frequent written communication from FDA about such things as the design of the proposed clinical trials and use of biomarkers
Eligibility for Accelerated Approval and Priority Review, if relevant criteria are met.
Rolling Review, which means that a drug company can submit completed sections of its Biologic License Application (BLA) or New Drug Application (NDA) for review by FDA, rather than waiting until every section of the NDA is completed before the entire application can be reviewed. BLA or NDA review usually does not begin until the drug company has submitted the entire application to the FDA.
NRXP NRX-100 is poised to address the >$3 billion Suicidal Depression market in the US.
Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA)
On August 8th NRXP announced it has received final clearance and approval from the Florida Agency for Health Care Administration (AHCA) to proceed closing of its Dura Medical LLC acquisition, in connection with its change of ownership applications, a key regulatory step for closing. Dura is revenue generating and EBITDA positive.
More on PrAtlas
- Melospeech Inc. Accepts Nomination for HealthTech Startup of the Year
- Flower City Tattoo Convention Draws Record Attendance in Rochester, NY
- KIKO NATION TOKEN (Official Release)
- Verb™ Presents Features Vanguard Personalized Indexing: Utilizing Advanced Tax-Loss Harvesting Technology
- UK Financial Ltd Announces A Special Board Meeting Today At 4PM: Orders MCAT Lock on CATEX, Adopts ERC-3643 Standard, & Cancels $0.20 MCOIN for $1
Request to the Food and Drug Administration Seeking Removal of Benzethonium Chloride from Ketamine Products to Favor a Switch to Better Options
On August 4th NRXP announced its actions with the US Food and Drug Administration (FDA), seeking the removal of Benzethonium Chloride from ketamine sold in the United States. Benzethonium Chloride (BZT) is a preservative that is not Generally Recognized as Safe (GRAS) by the FDA for parenteral products and not Generally Recognized as Safe and Effective (GRASE) for topical products. The FDA no longer allows BZT to be used in hand cleansers and topical antiseptics and other products on the market today for safety concerns. The ketamine versions made by NRXP is BZT free and therefore presents a safer and superior option.
Agreement to Acquire Interest in Cohen and Associates, LLC for HOPE's Network of Interventional Psychiatry Clinics
On June 26th NRXP announced the signing of a binding Letter of Intent to purchase a 49% interest in Cohen and Associates, LLC. Cohen is expected to serve as a foundational clinic for NRXP in the Sarasota-Bradenton region of western Florida.
Filing of Commissioner's National Priority Voucher Application for Intravenous Ketamine (NRX-100)
On June 23rd NRXP announced filing for the newly-announced FDA Commissioner's National Priority Voucher program on behalf of NRX-100, its patent-pending, preservative-free formulation of ketamine for intravenous use.
Strategic Investor Relations Partnership with astr partners
On June 16th NRXP announced a strategic investor relations partnership with astr partners, a boutique investor relations and capital advisory firm focused on the life sciences sector.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
Filed Under: Health, Banking, Biotech, Stocks, Financial, Finance, Medical, Marketing, Media, Healthcare, Stock Market, Nasdaq
0 Comments
Latest on PrAtlas
- $80M+ Backlog as Florida Statewide Contract, Federal Wins, and Strategic Alliance Fuel Next Phase of AI-Driven Cybersecurity Growth: Cycurion $CYCU
- High-Conviction CNS Disruptor Aiming to Transform Suicidal Depression, Ketamine Therapeutics, and TMS - Reaching Millions by 2030
- Top10Christmas.co.uk Releases the UK Christmas Toy Trends 2025 Report
- Talagat Business Academy Announces Joint Certificate Program With The University of Chicago Booth School of Business
- LocaXion and Asseco CEIT Announce First-to-Market RTLS-Driven Digital Twin Platform for Healthcare, Manufacturing, and Logistics
- Slotozilla Launches New Report on How AI Is Reshaping Careers and Society
- OKAVA Pharmaceuticals Announces First Cat Dosed in MEOW-1 Study of OKV-119, the World's First Clinical-Stage GLP-1 Weight-Loss Therapy for Pets
- Explosive Growth in U.S. Cryptocurrency Cloud Mining Sets The Stage for New Platform Launch with Daily Rewards in a Transparent Revenue-Share Model
- Qtex Cierra Ronda de $7 Millones para Estandarizar la Banca Transfronteriza en los Mercados Emergentes de Latinoamérica
- America's Most Festive Garages Wanted for Garage.com's 2025 Holiday Contest
- FDA Accepts ANDA for KETAFREE™ as Analyst Sets $34 Price Target for NRx Pharmaceuticals: (N A S D A Q : NRXP) NRx is Poised for a massive Breakthrough
- BEC Technologies Expands MX-220 5G Industrial Router Series for Edge Connectivity
- "Latino Leaders Speak: Personal Stories of Struggle and Triumph, Volume II" Documents the Truth About Latino Excellence and Impact on American Society
- Broadway Smile Boutique Unveils Modern Website for Enhanced Patient Experience
- Fenix Consulting Group Expands Orange County Office to Meet Growing Client Demand
- Signature Smiles Dental Group Unveils New User-Friendly Website
- CCHR: New Data Shows Millions of U.S. Children Caught in Escalating Psychiatric Polypharmacy
- QwickContractReview.com Launches $19 Contract Review Service to Protect Consumers from Hidden Contract Risks
- 100% Bonus Depreciation Places New Spotlight on Off The Hook Yacht Sales Inc. (N Y S E: OTH) as a Major Player in the $57 Billion U.S. Marine Market
- CNCPW Benchmarks Global Industry Standards: Integrating SEC Compliance with 3 Million TPS Architecture for Institutional Infrastructure